electron configuration for flourine|ground state electron configuration fluorine : Manila How to Write the Electron Configuration for Fluorine. Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in . FotonovelasXXX is an exclusive site for porn comics of the type fotonovelasxxx (real actors). This site does not contain Mangas online, hentai, 3d porn comics or similar comics but incest comics (fictitious erotic stories), Comix BDSM and Porn Stories with illustrative photos and much more material that you will not find in any other porn or .
electron configuration for flourine,How to Write the Electron Configuration for Fluorine. Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in . Mar 23, 2023 Fluorine's atomic electron configuration is 1s 2 2s 2 2p 5 (see Figure 2). Figure 2: Electronic configuration of fluorine. Fluorine is the most electronegative element . Fluorine Electron Configuration - YouTube. Wayne Breslyn. 766K subscribers. Subscribed. 325. 80K views 10 years ago. A step-by-step description of how to write the electron configuration.Electron configuration. The arrangements of electrons above the last (closed shell) noble gas. Melting point. The temperature at which the solid–liquid phase change occurs. . Electronic Configuration For Fluorine ion. The electronic configuration for the Fluorine ion is 1s22s22p5 and in this configuration, Fluorine needs 1 electron so as to complete the 2p orbital. This 1 .The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. By knowing the electron configuration of an element, we can predict .
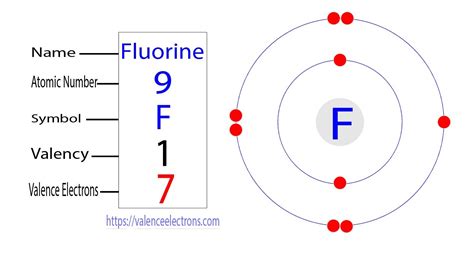
The electronic configuration of fluorine is: 1s2 2s2 2p5. Fluorine has 9 electrons arranged in two shells around the nucleus. The first shell contains two .
Elements in any one group (or column) have the same number of valence electrons; the alkali metals lithium and sodium each have only one valence electron, the alkaline earth .Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal .
One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s 2 2s 2 2p 4 electron configuration. Because all the 2p orbitals are degenerate, it doesn’t matter which one has the pair of electrons. Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5 and the orbital diagram is:
ground state electron configuration fluorine Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Fluorine (F) [He] 2s 2 2p 5: 1s 2 2s 2 2p 5: 2, 7: 10: Electron configuration of Neon (Ne) [He] 2s 2 2p 6: 1s 2 2s 2 2p 6: 2, 8: 11: Electron . In this video we will write the electron configuration for F-, the Fluoride ion. We’ll also look at why Fluorine forms a 1- ion and how the electron configur. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the . Fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure.The chemical symbol for Fluorine is F. Electron Configuration and Oxidation States of Fluorine. Electron configuration of Fluorine is [He] 2s2 2p5. Possible oxidation states are -1. Electron Configuration

In accordance with Aufbau principle, the step-by-step process of writing electron configuration is as follows: Step 1: Shell numbers are written in the first step. It has been identified that one Fluorine atom has 3 electron shell. The writing sequence of shell numbers would be 1, 2 and 3. Step 2: In the second step the orbitals are written .Element Fluorine (F), Group 17, Atomic Number 9, p-block, Mass 18.998. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element .

The electronic structure (configuration) of fluorine can be written as 2,7. In a similar way give the electronic configuration of: Given the fluorine atom has mass number 19 and electronic configuration 2,7. Find the number of protons, number of neutrons, number of electrons, and mass number. The number of electrons in an atom of Fluorine is 9.electron configuration for flourine ground state electron configuration fluorine Solution. 1. Locate the atom on the periodic table. 2. Locate the noble gas element in the period above the element of interest. 3. Continue the electron configuration from the noble gas until you reach the element of interest. 4. Put the noble gas in brackets and write the remainder of the electron configuration. The EA of fluorine is –322 kJ/mol. When we add an electron to a fluorine atom to form a fluoride anion (F –), we add an electron to the n = 2 shell. The electron is attracted to the nucleus, but there is also significant repulsion from the other electrons already present in this small valence shell.For hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (Figure 6.8.1 6.8. 1 ), and the electron configuration is written as 1 s1 and read as “one-s-one.”. A neutral helium atom, with an atomic number of 2 ( Z = 2), has two electrons. We place one electron in the orbital that is .electron configuration for flourine 1 Answer. F −:1s22s22p6. Elemental Fluorine has an electron configuration of 1s22s22p5 and needs 1 more electron to complete its 2p orbital which it will acquire in formation of the fluoride ion. Thus it gains an electron when forming the fluoride ion, and becomes isoelectronic to neon. F^- : 1s^2 2s^2 2p^6 alternatively: F^- : [Ne] Elemental .
Electron configurations of ions. To find the electron configuration for an ion, first identify the configuration for the neutral atom. Then, add or remove electrons depending on the ion's charge. For example, to find the configuration for the lithium ion (Li⁺), start with neutral lithium (1s²2s¹). Then, since the lithium ion has one less . The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the .
Similarly, fluorine has the electron configuration 1s 2 2s 2 2p 5: When we reach neon, with Z = 10, we have filled the 2p subshell, giving a 1s 2 2s 2 2p 6 electron configuration: Notice that for neon, as for helium, all the orbitals through the 2p level are completely filled. This fact is very important in dictating both the chemical . The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the .
electron configuration for flourine|ground state electron configuration fluorine
PH0 · the correct electron configuration for te is
PH1 · shorthand electron configuration for fluorine
PH2 · ground state electron configuration fluorine
PH3 · electron configuration for every element
PH4 · electron configuration explained
PH5 · electron configuration calculator
PH6 · complete the electron configuration for f
PH7 · complete electron configuration for carbon
PH8 · Iba pa